Is Licl an Acid or Base
Chemistry questions and answers. LiCN Lithium cyanide is base What is an acid base neutral.

Salts And Ph Soluble Salts Dissociate In Water To Produce Ions Salts Are Basically Ionic Compounds That Can Be Formed From The Reaction From An Acid Ppt Download
Determine whether each of the following salts will form a solution that is acidic basic or pH-neutral.

. Determine whether each substance is Acidic basic or neutral. Potassium chloride KCl is a water-soluble compound that is widely used to prevent or treat various etiologies of severe potassium loss Hypokalemia or severe. To tell if LiCl Lithium chloride forms an acidic basic alkaline or neutral solution we can use these three simple rules along with the neutralization r.
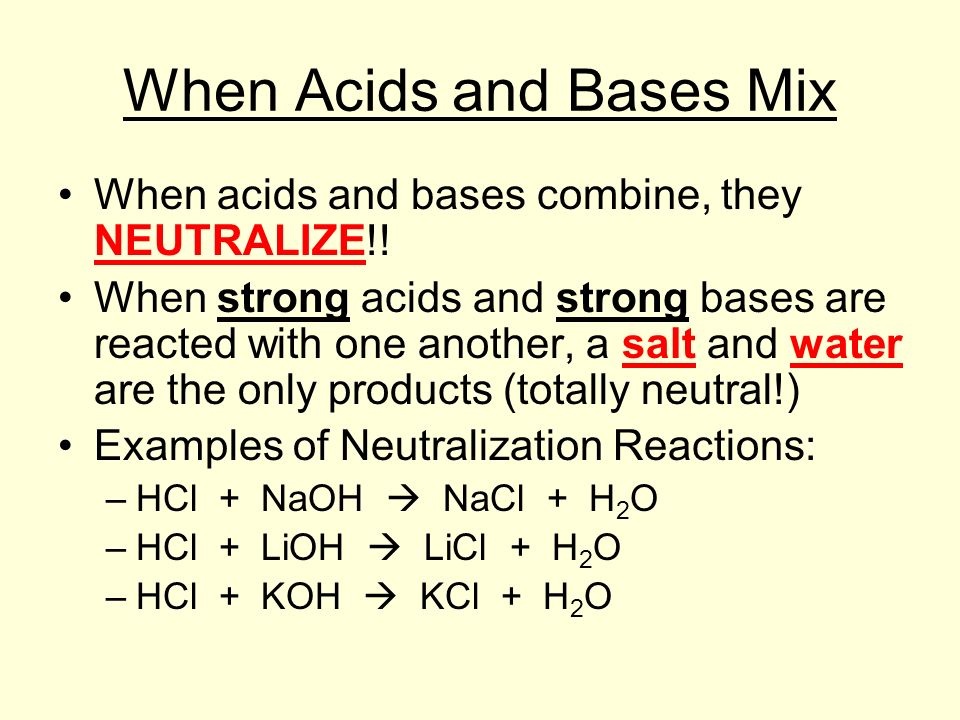
Classify the compounds as acids bases or salts о Acid Base Salt HF LiCl NHBr CsOH Ca OH2 H2SO MgBr Answer Bank. It is made from the neutralization reaction of the strong acid namely hydrochloric acid HCl with a strong base namely potassium hydroxide KOH. The KCl ions come from a strong acid HCl and a strong base acid HCl KOH.
RbCl LiCl C5H6NCl C5H5N is the weak base pyridine K2S KNO3 Sodium benzoate K3PO4 NaCN. Dear student CaCl2 is a salt of strong acid HCl and strong base CaOH2. What is the net ionic equation for the reaction of formic acid HCOOH with NH3.
We can use K a and K b values to determine the weaker acidbase. So does this mean AlCl3 is a weak base since its conjugate acid is strong. Start studying Strong Acids Bases Exam 2.
Sulfuric acid and lithium hydroxide B. Because strong acid and a strong base will neutralize each other effects and a neutral solution forms. -What is the pH of a 0100 M formic acid solution with a K a 18 x 10 4.
A reaction between an acid and a base produced lithium chloride LiCl. LiCl nuetral AlNO 3 3 acidic BaF 2 basic NH 4 I acidic 6. So on keeping it is bound to hydrolyse into its parent components viz LiOH and H2CO3.
Learn vocabulary terms and more with flashcards games and other study tools. C5H6NCl C5H5N is the weak base pyridine K2S. According to the lewis theory a compound is said to be acid when it accepts the pair of electrons and a compound is said to be base when it donates the pair of electrons.
Since LiOH is a strong base the. In this context the term neutral refers to an equalibrium point which is. As a result of this reaction the solution will be acidic with a pH 7.
Is Licl An Acid Or Base Or Neutral Thus the acidity of the solution will not be influenced by either ion so KCl is a neutral salt. It is obtained by the reaction of a strong acid and base. Thus it forms a neutral solution when dissolved in water.
From the reaction AlCl3 3H2O AlOH3 3HCl HCl is a strong acid so it will dissociate in water to form H and Cl-. Hydrochloric acid and lithium hydroxide C. AlClO43-Acid LiCl-Neutral KClO2-BAse C6H5NH3NO2-Acid CH3NH3Br-Acid KCl-Neutral NaClO-Base NH4ClO-Acid FeClO43-Acid.
Sulfuric acid and magnesium hydroxide D. Thus the acidity of the solution will not be influenced by either ion so KCl is a neutral salt. It is a salt formed by the neutralization reaction between an acid and a base.
Figure out which acid and base would make the salt in this case FeOH3 and HCl2. To determine whether a salt is acidic or basic try the following1. What acid and base combination could produce this salt.
Yes it is a Lewis acid. Determine whether each substance is Acidic basic or neutral. However in solution it is bound to be basic since it is the salt of a strong base Lithium hydroxide and weak acid carbonic acid.
The pH value of the aqueous solution of KCl is 7. It means Lewis acid is a compound that has a deficiency of electrons thats why they are electron-pair acceptors and lewiss base is a compound that is rich in electrons so they are electron-pair. AlClO43-Acid LiCl-Neutral KClO2-BAse C6H5NH3NO2-Acid CH3NH3Br-Acid KCl-Neutral NaClO-Base NH4ClO-Acid FeClO43-Acid chemistry Strong base is dissolved in 665 mL of 0400 M weak acid Ka 369 10-5 to make a buffer with a pH of 394.
What does it mean. The equilibrium of a BronstedLowry acidbase reaction will favor the side of the equation with the weaker acid and weaker base. Hydrochloric acid and magnesium hydroxide.
Is lithium chloride an acid or base.
The Ph Scale The Measure Of The Difference Between Acids Bases And Salts Ppt Download

No comments for "Is Licl an Acid or Base"
Post a Comment